Lab Design in the Time of COVID
Modular planned lab with BSL-2+ suites.
PHOTOGRAPHY BY ANTON GRASSL FOR HGA
By: Mark Allen, AIA, LEED AP
Insights into science have always been the key to understanding how the design for a lab can best support the scientists working there. COVID-19 has brought this view into sharp focus.
Lab design for life sciences organizations is being impacted as a result of COVID-19, and 2021 will be the year that urgent design responses to the pandemic coalesce into trends. In many cases, the impact of COVID-19 on lab design builds on shifting space planning and space requirements that were evident prior to the pandemic. The pandemic has amplified trends in modular planning and the mix of lab requirements.
From a lab and facility design perspective, the life sciences ecosystem addressing COVID-19 can be divided into five categories:
genome sequencing—tracking mutations of the virus
diagnostics—such as PCR and antigen tests
vaccine and therapy research
vaccine and drug development for clinical trials
manufacturing of vaccines and therapies
Except for large-scale manufacturing, much of the above work will take place in lab environments that need flexibility to adapt to unknowable future demands. RT-PCR testing, for instance, is the current U.S. standard based on its accuracy and is still on a growth curve in volume. When will the need for this type of pandemic testing abate, and how quickly will facilities be able to pivot to new demands?
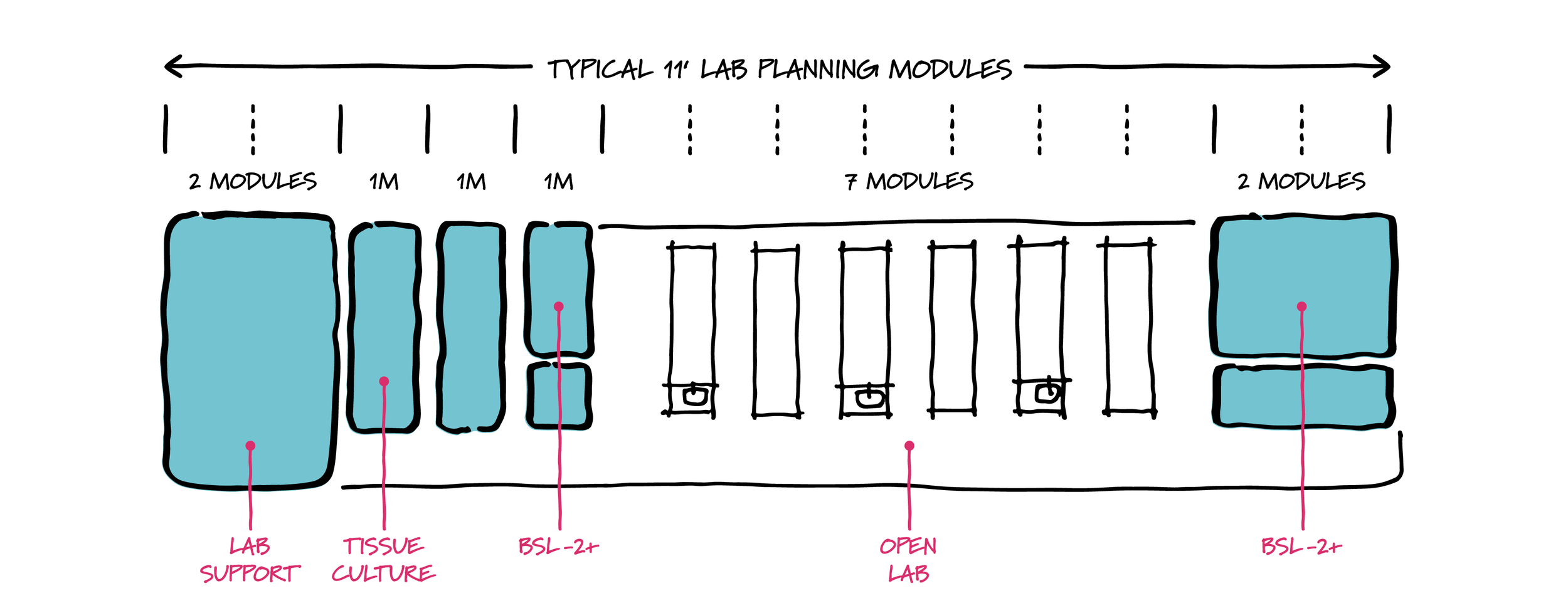
Modular lab planning considerations.
HGA
Commercial and institutional organizations with relevant lab expertise have been quickly reconfiguring parts of their facilities to respond to the pandemic. Labs with capabilities in automated liquid handling and high-throughput PCR processes have been converting spaces into COVID-19 diagnostic labs. But testing will likely decline at some point in the near term, and could do so rapidly if other faster, less expensive, or at-home tests demonstrate similar accuracy. Maintaining the ability to unwind some specialty COVID-19 diagnostic labs and return them to productive research and other uses will become an essential lab design task by the end of 2021. Fortunately, there are several parallels between the environments that support COVID-19 work and other life sciences targets.
The race for vaccines and therapies to address COVID-19 has highlighted the growing importance of biologics vs. traditional small molecule pharmaceutical drug development. The daily reference in general media to monoclonal antibody treatments, viral vector vaccines, and messenger RNA technologies is one marker for this trend. These technologies represent the promise that biologics will be the key to bringing the pandemic under control, and the FDA has committed to expediting clinical trials of biological products for COVID-19 prevention or treatment. While analytical chemistry has a role, the small molecule pharmaceuticals based on chemistry screening and synthesis are not yet proven in meeting this challenge. Beyond COVID-19, there are wider parallels with drug development in general. With some exceptions, life sciences research programs are focusing on more lab space suited to a diverse range of biological work and less space dedicated to chemistry-intensive processes.
A different kind of robust
Modular lab planning.
HGA
The shift from space requirements that previously favored chemistry-intensive programs has deep impacts on architecture and design. Large volumes of flammable solvents and hazardous chemicals once restricted lab occupancy to lower floors in a structure, as required by building codes. In contrast, the lower volumes associated with biologics and similar work allows labs to be located on higher floors of research buildings.
Previous demands for extensive exhaust systems across a floorplate are now substantially reduced in biologics labs with many fewer chemical fume hoods, and the compartmentalization to accommodate large volumes of solvents is often much less. In its place are new requirements for emergency power at high densities on instrumentation, liquid handlers, and ultra-low temperature freezers—the tools for both COVID and non-COVID related labs.
Flexibility is paramount in this new lab design paradigm. The ability to pivot labs to new demands as current priorities fade will be based on more modular planning. A central philosophy of modular lab planning is that floorplates are organized on a regular module (often 11 ft. x 30 ft., with many variations) so that open labs and lab support can expand and contract without major modifications. A planning module accounts for the space of the lab bench, the open aisle behind it, and the lab bench on the other side of the aisle. The module should be flexible to allow a range of scenarios—such as replacement of a lab bench with a chemical fume hood, or separation of spaces with walls for a tissue culture room and other lab support.
Mechanical, electrical, and plumbing systems set to this regularized module are equally important. Lab gasses, for instance, can be provided at regular intervals in ceiling service panels to anticipate the services of future lab instruments. COVID-19 is a stress test on labs; spaces designed with modular lab planning will be the ones that can quickly adapt to new uses.
More viral vector work
One of the COVID-19 vaccines in production uses another virus as a delivery system. This type of viral vector vaccine delivers genetic instructions to our cells to manufacture COVID-19 spike protein—triggering an immune response and the production of protective antibodies.
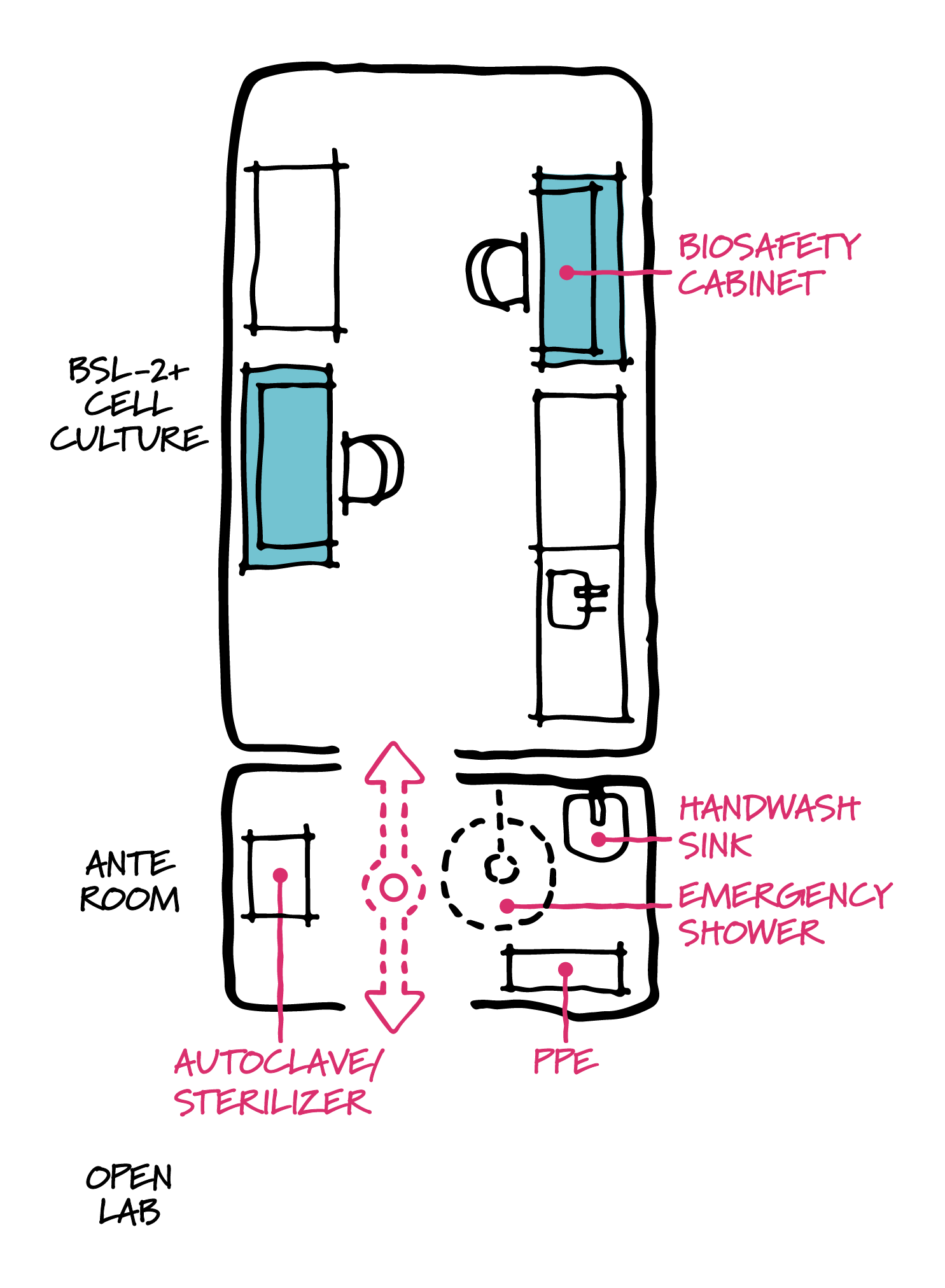
BSL-2+ diagram.
HGA
How does this affect lab design? Protocols for safe handling of these types of viruses generally prohibit work on open benches in the lab. Additional safety considerations include biosafety cabinets for manual processes, as well as additional safety considerations. To address this growing need, many research programs and institutions have developed standards for dedicated BSL-2+ suites—environments with features and protocols more advanced than NIH Biological Safety Level 2 (BSL-2) labs most typical to biotech and pharmaceutical research, though not as onerous to build and operate as labs designed for aerosolized pathogens (typically BSL-3).
With the increase in research on a broad array of pathogens in addition to COVID-19, we are also likely to see a demand for BSL-3 containment labs. There may be a countertrend here too. As BSL-3 requires dedicated exhaust systems, personnel training, and protocols that are significant, there is an increasing interest in BSL-2+ lab design that satisfies specific research needs in a less demanding environment. While there is no single defined standard, BSL-2+ suites may include ante room vestibules for entering/exiting personnel to don dedicated personal protective equipment (PPE), wash hands, and sterilize lab waste. These suites are also good candidates for modular lab planning: one suite = one lab module, or double wide = two lab modules.
More cell and gene therapy suites
We will see increasing use of this type of lab suite configuration in 2021 for additional reasons. The molecular biology revolution built around genomic DNA sequencing and RNA sequencing is beginning to see long-promised advances come to fruition. Messenger RNA vaccines for COVID-19 are the most notable example. Sequencing and the gene editing techniques are also the bases for FDA-approved gene therapies to treat lymphoma and retinal dystrophy (which causes blindness), with several hundred other gene therapies currently in clinical trials. COVID-19 will drive increases in sequencing volume for other reasons as well: researchers use sequencing to examine the mutation of the virus—key to tracing its path and to determining whether today’s vaccines and treatments will continue to be effective.
Lab processes leading up to the sequencing of DNA and RNA are sensitive to contamination. The suites that many labs dedicate to the extraction and amplification of DNA and RNA are often equipped with ante rooms like those provided for viral vector work described above. As many gene therapies are also currently dependent on viral vectors, design of lab suites with ante rooms will continue to grow on both the genomics and viral vector fronts.
Compact footprints for clinical trials
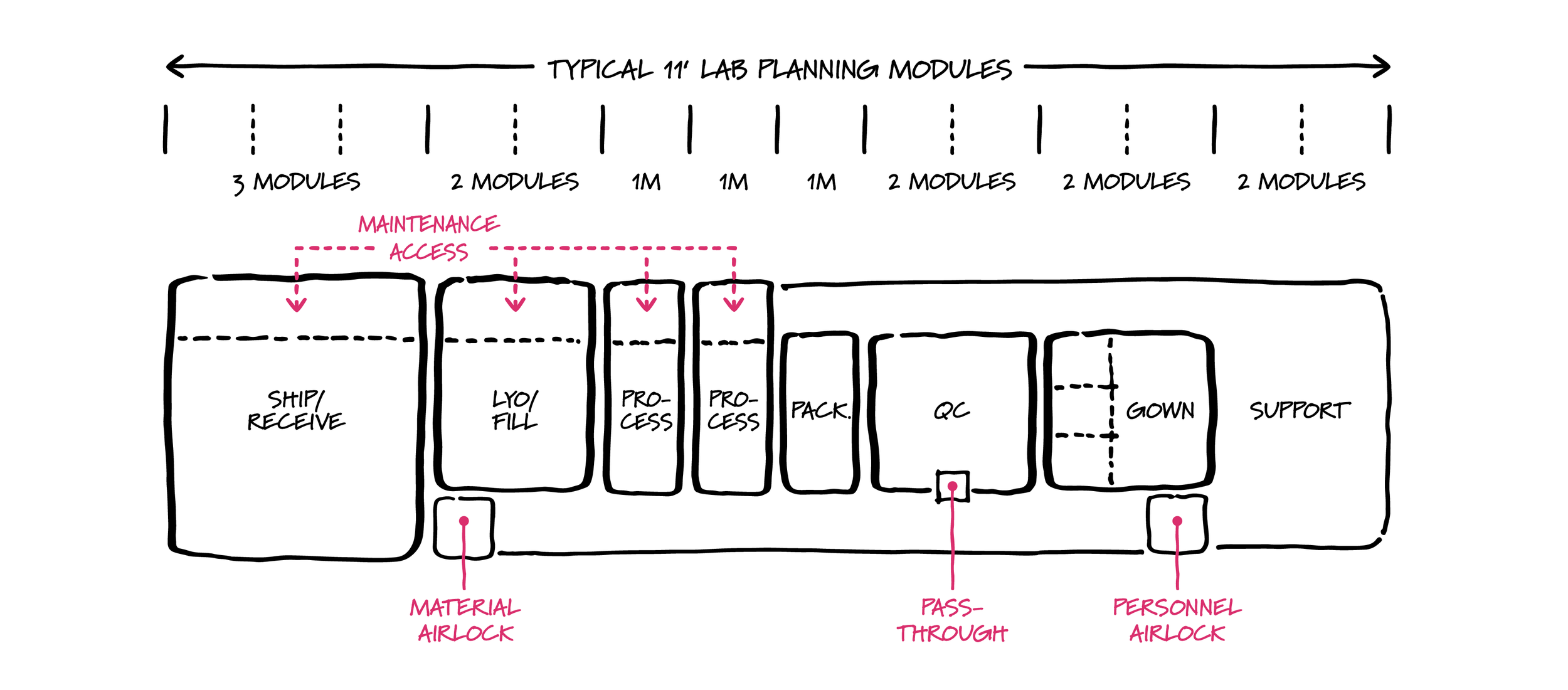
Modular lab planning applied to cGMP production.
HGA
Wide distribution of the current therapies and vaccines for COVID-19 will require large cGMP (current Good Manufacturing Practice) production facilities to manufacture massive volumes at low cost. However, there are parallel efforts underway for many additional therapies and vaccines to be produced in only the small volumes needed for pre-clinical and Phase 1 clinical trials. And what will the framework look like to support the pipeline for regenerative medicine, immunotherapy, and gene therapies for rare diseases? These types of work benefit from close connection of research lab to the small-scale production of therapies for clinical trials. Distance from the biotech research clusters and academic medical centers is a potential barrier to speed, innovation, and quality control.
Compact footprint production suites constructed adjacent to the lab help solve the issue of proximity. Given the often-high cost of space and construction in research clusters and medical centers, it is essential to optimize space efficiency with these footprints—another good candidate for modular lab planning.
With futureproofing in mind, the spatial components of production suites can be organized on lab planning modules, allowing space for expansion to meet a different regulatory requirement, material flow, or process. This also allows for production, process development, and research lab areas to ebb and flow with the demands of production.
Conclusion
COVID-19 is amplifying existing research and production trends. Genome sequencing and PCR processes are two such growth areas that are now gaining even more momentum. Will we need to unwind some of this capacity post-Covid? And will organizations continue to expand techniques for viral vectors and related work? While the specific needs may be unknown, modular planning and flexible lab design will be critical in pivoting to new research targets and future therapies.
Mark Allen, AIA, LEED AP, is principal at HGA.